- HOME
- Research and Development
- Research Contents
Research Contents
In-house pipeline:
Pitavastatin nanoparticle preparation:

Nanoparticles of the anti-inflammatory drug pitavastatin are expected to selectively deliver the drug to vascular endothelial cells, induce angiogenesis, suppress the progression of pulmonary arteriole lesions, and lead to healing. Through our research and development so far, we have completed the physician-initiated Phase 1/2a clinical trial for severe ischemic limbs and the physician-initiated Phase 1 clinical trial for severe pulmonary hypertension. Utilizing the above data and our experience, we are also developing new therapeutic agents for other diseases. Since our technology is well-established, non-clinical data can be used to accelerate clinical development and greatly reduce costs. We have already started new joint development with academia and a global veterinary-drug manufacturer.
The 2-deoxy-D-glucose (2DG) nanoparticle preparation

Although 2DG mediates its anticancer effect through glycolysis inhibition, it exhibits severe side effects, such that clinical development has been suspended. We developed a 2DG nanoparticle formulation in collaboration with the Kawasaki Medical School. It has been confirmed that the formulation reduces the side effects of 2DG, improves tumor-suppressive action, and activates anti-tumor immunity; the formulation is now under development as an epoch-making anticancer therapeutic agent.
Nanoparticle Adjuvant
Through joint research with Osaka University, we successfully formulated a nanoparticle-based vaccine adjuvant.
It has been shown to enhance the immune activity of the spike protein that constitutes SARS-CoV-2, the novel coronavirus.
It has been shown to enhance the immune activity of the spike protein that constitutes SARS-CoV-2, the novel coronavirus.
Development of a new vaccine that fulfills the SENTAN platform offers the following:
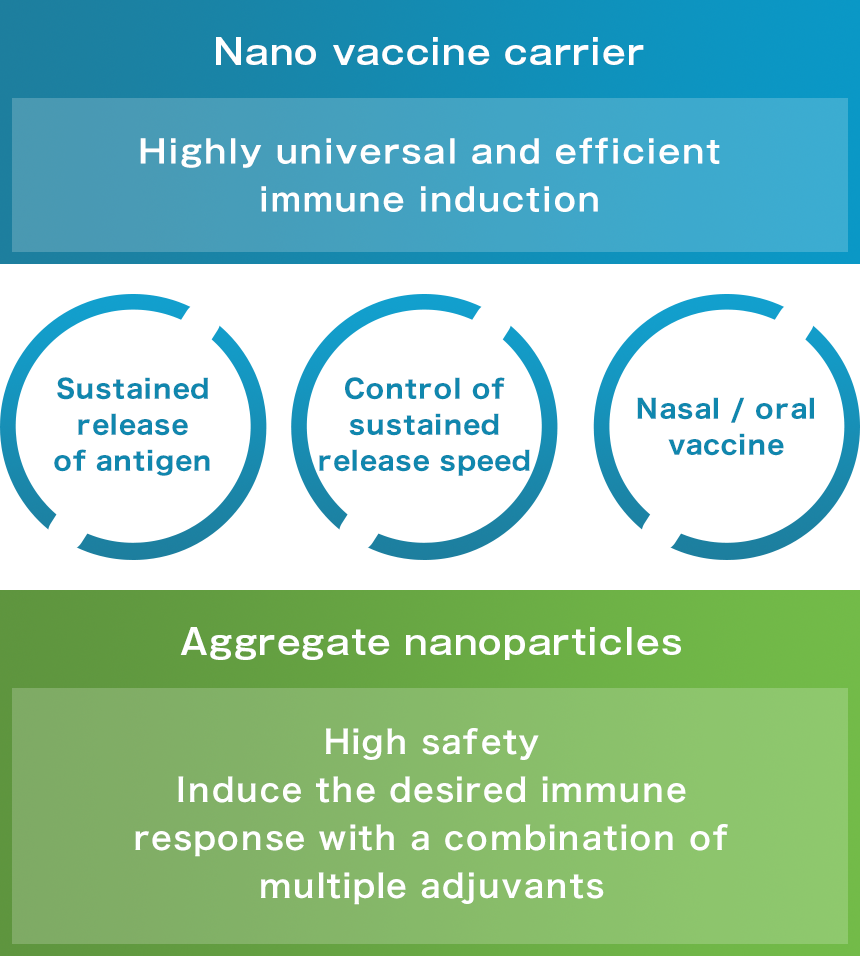
for example
● There are no highly effective vaccines for HIV, malaria, tuberculosis, etc.
● New threats such as COVID-19, SARS, and new influenza| Vaccines / adjuvants Development issues |
Safety Efficacy of a new vaccine with no allergies or side reactions Efficacy
Insufficient type of adjuvant Convenience
Minimally invasive vaccine (nasal alternative to injection, oral) Pandemic compatible
Responding to new threats, mass production |
|---|
Development of next-generation inhalant applying nanoparticle technology

We are developing next-generation inhalation preparations for new coronavirus, influenza, pneumonia, etc.
■ aiming for high local lung concentration and sustainability by inhalation formulation
■ suppress virus "invasion" into the lungs ⇒ preventing virus replication in cells
Features of SENTAN Pharma "Primary Particles"
■ delivery of drugs deep into the lungs
■ stronger adhesion to the lungs' surface leads to long-lasting drug effects
■ reduction of local irritation
■ technology applies not just to COVID-19 but also to other disease indications because it is not limited by the drug choice.
■ stronger adhesion to the lungs' surface leads to long-lasting drug effects
■ reduction of local irritation
■ technology applies not just to COVID-19 but also to other disease indications because it is not limited by the drug choice.
Co-development pipeline:
We will create a new pipeline of drug discovery and preventive medicine based on the results of joint research and development utilizing our nano-microparticle technology.
Joint research and development


